NEWS UPDATES
Sunday, December 18, 2016
Saturday, December 17, 2016
The Snamprogetti Urea Process Description
0
8:29 PM
The Snamprogetti Urea Process Description
The Snamprogetti Urea Process is basically a total recycle stripping
process using ammonia as self stripping agent. The Snamprogetti process
used the excess ammonia present in the urea solution leaving the reactor
to strip CO2 from urea solution in a falling film steam heated heat exchanger operated at the urea reactor pressure. The separated CO2 and NH3
are recombined as ammonium carbamate in the carbamate condensers, also
operating at urea reactor pressure and then returned to the reactor for
conversion to urea.
The overall result of this scheme is that an internal recycle of both NH3 and CO2 in the urea reactor system is established without having to pump either component , as in previous total recycle process in which NH3 and CO2 were separated from the solution at lower pressure. This substantially reduces the high pressure pumping requirements for both the NH3 and ammonium carbamide solution, since in the Snamprogetti stripping process approximately 80% of the CO2 fed is converted to urea within high pressure synthesis loop and only about 20% of it must be pumped back to the reactor as ammonium carbamate solution from lower pressure. In addition to the reducing pumping requirements, this process permits a substantial saving in steam requirement, since the operating temperature level of the ammonium carbamate condenser by utilizing the heat released by the condensing vapor.
The NH3 to CO2 ratio used in Snamprogetti process is 3.3-3.6 : 1which combines with a temperature of 186-189deg C and pressure of 155 Kg/cm2g approximately, permits a conversion yield in the reactor of 62 to 65%. Plant layout

The overall result of this scheme is that an internal recycle of both NH3 and CO2 in the urea reactor system is established without having to pump either component , as in previous total recycle process in which NH3 and CO2 were separated from the solution at lower pressure. This substantially reduces the high pressure pumping requirements for both the NH3 and ammonium carbamide solution, since in the Snamprogetti stripping process approximately 80% of the CO2 fed is converted to urea within high pressure synthesis loop and only about 20% of it must be pumped back to the reactor as ammonium carbamate solution from lower pressure. In addition to the reducing pumping requirements, this process permits a substantial saving in steam requirement, since the operating temperature level of the ammonium carbamate condenser by utilizing the heat released by the condensing vapor.
The NH3 to CO2 ratio used in Snamprogetti process is 3.3-3.6 : 1which combines with a temperature of 186-189deg C and pressure of 155 Kg/cm2g approximately, permits a conversion yield in the reactor of 62 to 65%. Plant layout
Block diagram of total recycle of ammonia stripping in urea production:

Urea production takes place through the following main operations:
- Urea synthesis and high pressure recovery.
- Urea purification in the medium, low pressure decomposers, pre-vacuum concentrators. Urea concentration.
- Urea Prilling.
The liquid ammonia coming from the battery limit at a temperature of 12oC
and 18 Kg/cm2(g) is collected in a ammonia receiving vessel
which is operated at a medium pressure(17Kg/cm2). From the ammonia
receiver, the liquid ammonia is pumped to the reactor by two pumps. The
first pump is the ammonia booster pump which is centrifugal type and
supplies the liquid ammonia at 22Kg/cm2 to second pump suction. The
second pump is the high pressure ammonia pump which is a
reciprocating-plunger type. Due to the reciprocating motion of second
pump pulsating takes place in the discharge of booster pump and those
cause the change of loads on booster pump to avoid these booster pump
and those cause the change of
loads on booster pump to avoid these pulsations a damper is provided in
the suction of second pump. In the H.P. ammonia pump, the ammonia
pressure is increased to 239Kg/cm2 and then goes to a ammonia pre heater where it is preheated to 75oC
by L.P. decomposer outlet gases, H.P. ammonia is used as pressure
section contains first and second stage another one is the high pressure
section contains third and fourth stage. First and second stages
contains three, third stage contains four and fourth stage two numbers
of closed type impellers. Carbon dioxide gas enters first stage suction
at 1.5kg/cm2 and 40oC and compressed to 160 Kg/cm2
in four stages. Intercoolers are provided after first, second and third
stages to cool the carbon dioxide using cooling water. Along with
coolers knock out drums are provided between each stage in which
moisture gets separated. Lube oil system provides the lubrication of the
rotating parts. The superheated steam and saturated LS steam is used as
a driving fluid for steam turbine. Superheated HS steam is extracted
and part of steam is condensed in condenser. Condensate is then pumped
to D.M. plant.
 |
| Flow sheet of Urea prilling system |
 |
| Block diagram of urea prilling section |

NITRIC ACID Production Process
0
8:28 PM
NITRIC ACID Production Process
Properties of NITRIC ACID
1. Appearance Colorless to yellowish liquid
2. Odor Suffocating, acrid
3. Solubility Infinitely soluble
4. Molecular weight 63.013
5. Boiling point 86°C
6. Viscosity 1.62cp
7. Density (60% conc.)
- 0°C 1.3931Kg/m3
- 25°C 1.36 Kg/m3
- 100°C 1.2547 Kg/m3
09. Specific gravity 1.502
10. Critical temperature 520°K
11. Critical pressure 68.9 bar
12. Critical volume 145 cm3/mol
13. Diffusivity in the water 2.9X10-5 cm2/s
14. Vapor Density (Air=1) 2-3 Kg/m3
15. Vapor Pressure (mm Hg) 48 @ 20°C (68 °F)
16. Specific heat (20°C) 0.64 cal/g
Classification of Nitric Acid Production Processes:
2. NaNO3 +H2SO4 process (Chile Salt Peter process)
3. N2 fixation from air (Wisconsin process)
4. Nitrogen fixation by nuclear fission fragments
OSTWALD PROCESS
Reactions involved in the Ostwald’s process
Main reactions
1. Oxidation of NH3 to NO
1. Oxidation of NH3 to NO
NH3+5/4O2 →NO+3/4H2O ∆H= -54Kcal
2NO+O2 → 2 NO2 ∆H= -27.2Kcal
3. Absorption of NO2 in water
3. Absorption of NO2 in water
2NO2+H2O → HNO3 +HNO2
4. Concentration of HNO3
4. Concentration of HNO3
Side reactions
NH3+3/4O2 → 1/2N2+3/2H2O ∆H =-75.7Kcal
NH3 → 1/2 N2 +3/2H2
NH3+O2 → 1/2N2 O+3/2H2O
NH3+3/2NO → 5/4N2 +3/2H2O ∆H= -107.9Kcal
Raw materials required for manufacture of nitric acid
a. Anhydrous Ammoniab. Filtered air
c. Platinum –Rhodium catalyst
d. Water
Anhydrous Ammonia
Medium temperature (~500 degC)
Very high pressure (~250 atmospheres, ~351kPa)
A catalyst (a porous iron catalyst prepared by reducing magnetite, Fe3O4).
Osmium is a much better catalyst for the reaction but is very expensive.
This process produces an ammonia, NH3 (g), yield of approximately
10-20%. The Haber synthesis was developed into an industrial process by
Carl Bosch. The reaction between nitrogen gas and hydrogen gas to
produce ammonia gas is exothermic, releasing 92.4kJ/mol of energy at
298°K (25degC).
Platinum-Rhodium catalyst
Properties of platinum- Atomic number:- 78
- Atomic weight:-195.09g./mol
- Density:-21.45gm/cm3
- Melting point:-1769°C
- Boiling point:- 3827°C
- Thermal conductivity :-73 Watt/meter/°c
- Tensile strength:- 14kg/mm2
- Isotopes:-6
- Electrical resistivity:- 9.85 micro hg.cm at°C
Chemical properties
It has the third highest density behind osmium and iridium
Platinum is unaffected by air and water but will dissolve in hot aqua regia, in hot concentrated phosphoric and Sulphuric acid in the molten alkali
It is as resistant as gold to corrosion and tarnishing. Indeed, platinum will not oxidize in air no matter how strongly it is heated.
Properties of Rhodium
Rhodium is silver white metal
Melting point:-1966°C
Boiling point:-4500°C
Density:- 12.41 gm/cm3
Special properties
High electrical and Heat conductivity. That means heat and electricity pass through rhodium easily
Chemical properties
Is relatively in active metal. It is not attacked by the strong acids. When heated in air, it is combined slowly with O2.
Main components involved in the process are:
- Kobe air compressor
- Secondary air compressor – 1and 2
- Instrument air compressor-A,B
- Air drying unit
- Air receiver
- Silica gel for dry air
- Turbine
- Catalytic converter
- Air-heater
- Oil separator
- Ammonia evaporator
- Ammonia super heater
- Air – ammonia mixer
- Mixed gas filters -1 and 2
- Waste heat boiler (W.H.B)
- Deaerator
- Tail gas heater -1,2 and 3
- Boiler feed water (B.F.W)
- Start acid up tank
- Absorption tower
- Bleaching tower
- Product acid cooler
- Storage tank
Air Compressor and Turbine:
Air from atmosphere is suck at ambient temperature (room temperature)
into the compressor. The compression is done in three stage driven by
electric motor and turbine which is in turn run by tail gases .The air
first passes through 1st stage at room temperature and leaves at
temperature of 130°C and pressure of 2.02kg/cm2.This is then cooled in
the inter cooler to 55°C by using raw water as cooling medium. Then air
enter the second stage where it is compressed to 3.5kg/cm2 and
temperature of 118°C and after the second stage is cooled to 70°C in
the inter cooler then it enters the third stage where it is compressed
to 4.5kg/cm2 abs and outlet temperature of 143°C. 40% of the energy
required for carrying out compression operation is supplied by tail gas
turbine. These tail gases are generally taken from absorption tower
which leave at 19°C.it is heated up to 260°C by the series of heat
exchangers. Major amount of air which is called as a primary air is sent
to Air-heater.
Secondary Air Compressor-1, 2:
The secondary air which is supplied by secondary air compressor-1, 2 used is in the bleaching tower
Air-Heater:
The air from the compressor enters the air-heater at 143°C and there it
is further heated to 208°C by using high pressure steam and leaves at
208°C.
Ammonia Evaporator:
The liquid ammonia stored in ammonia bullets is sent to the evaporator
at 10-12kg/cm2 through tube side and 21°C temperature, where it is
vaporized by the chill water coming from the absorption tower passing
through shell side. The ammonia leaves the evaporator at 19°C.
Ammonia Super Heater:
The ammonia which enters the super heater is further heated to 80°C by
using low pressure steam. Here the shell side flow is ammonia and the
tube side flow is low pressure steam.
Ammonia-Air Mixture:
Ammonia enters the mixer at 80°C and air at 208°C and mixing takes place
and heat is exchanged between them and leaves at 180°C. proportion of
Ammonia and Air is10-10.7.
Mixed Gas Filter 1, 2:
Mixed gas filters consists of “SS –candle” as filter medium. The air
–ammonia mixer enters this filter in order to remove the impurities
present in the mixture. Presence of the impurities in the mixture may
corrode the catalyst surface.
Waste Heat Boiler:
At the entrance of the waste heat boiler the mixture may be around
180°C. Here the hydrogen flame is used to raise the temperature. This
waste heat boiler consists of platinum-rhodium catalyst for the reaction
to start. First it consists of supporting bars on which the nichrome
mesh is placed, above which the palladium catchment gauge is placed and
finally the platinum (95%)-rhodium (5%) catalyst is placed. The reaction
is carried at 850°C. At the bottom part of the waste heat boiler which
is in the form of cylinder, consists of tubes in which the NOX gases
flow and on shell side boiler feed water is supplied for cooling the NOX
gases. The heat that which is produced by the NOX gases is gained by
the boiler feed water and high pressure steam is generated. This high
pressure steam is sent to steam generation station where it is split
into low pressure, medium pressure and high pressure steam. They are
utilized in some parts of the plant. Here the rich gases is sent into
the tail gas heat exchanger III and the lean gases is sent into tail gas
heat exchanger-II
Tail Gas Heat Exchanger-II:
Tail gas heater –II is a shell and tube heat-exchanger in which the
nitrous gas is passed through shell side which enters at 320°C and
leaves at 280°C and tail gas (coming from T.G.H-I) is passed through
tube side which enters at 160°C and leaves at 250°C. The tail gases are
sent to catalytic converter and the nitrous gases are sent to boiler
feed water.
Boiler Feed Water:
Here de-mineralized water which is de-aerated by steam and sent through
tube side is used for cooling the nitrous gas passing through shell
side. The inlet temperature of the nitrous gas is 280°C and outlet
180°C. The outlet steam is sent into waste heat boiler. The nitrous gas
is further sent to T.G.H-I.
Tail Gas Heater-I:
Tail gas heater –I is a shell and tube heat-exchanger in which the
nitrous gas is passed through shell side which enters at 180°C and
leaves at 155°C and tail gas (coming from Tail gas pre-heater) is passed
through tube side which enters at 45°C and leaves at 160°C. The tail
gases are sent to catalytic converter through T.G.H-II and the nitrous
gases are sent to condenser.
Cooler Condenser:
The nitrous gas from T.G.H-I is sent to the shell side of the cooler
condenser and the cold water from the VAM unit is circulated on the tube
side. The nitrous gas enters the cooler condenser at 155°C and leaves
at 56°C.The cooled water is recycled and the nitrous gas is sent the
adsorption tower.
Absorption Tower:
This tower consists of 69 trays in which 64 trays are absorption trays
and the 5 trays are oxidation trays. The nitrous gas are first sent to
oxidation trays there nitrous gas converted into NO2 and then it is sent
into the absorption trays in which the De-mineralized is sprayed from
top and the NO2 gets converted into HNO3 (nitric acid). Then it is
finally sent to bleaching tower. The tail gases are sent to tail gas
pre-heater
Tail Gas Pre-Heater:
The tail gas from the absorption tower is sent to the tail gas
pre-heater in which the tail gases are heated by using low pressure
steam. The tail gas enters the tail gas pre-heater at 19°C and leaves at
45°C. The outlet tail gas is sent to new tail gas heater-III and also
to T.G.H-I.
New Tail Gas Heater-III:
The rich gases from the waste heat boiler are circulated on shell side
of the new tail gas heater-III. This enters at 320°C and leaves at
160°C. The tail gas from the tail gas pre-heater is circulated on tube
side. This enters at 45°C and leaves at 285°C. Finally the tail gas from
the tail gas per-heater-III is used to run the turbine.
Bleaching Tower:
The secondary air from the secondary air compressor is supplied to the bleaching tower to remove the color of the nitric acid.
Product Acid Cooler:
The nitric acid thus obtained is cooled by using the cooling water in
the product acid cooler. Then it is finally sent to the storage tank-A, B
Catalytic Convertor:
Tail gases from the T.G.H-II are sent to catalytic converter and the vapor ammonia is also fed from NH3 super heater.
NO + NO2 +2NH3→ 2N2 + 3H2O
Here tail gases and ammonia reacts with each other and forms nitrogen
and water vapour. This can then be safely disposed to atmosphere
NITRIC ACID PRODUCTION FROM CHILE SALT PETER PROCESS:
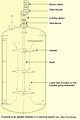
NaNO3 + H2SO4 → NaHSO4 + HNO3Reactor for Urea Production and Urea Process Parameters
0
8:27 PM
 |
| Low Pressure Decomposer |
 |
| Diagram of Low Pressure Urea Section |
A complete urea process description with flow sheet
Brief equipment design of a reactor for producing 2100 MTPD of Urea:
Internal trays :
| Sieve trays | : 480 holes 8 mm diameter, equispaced triangular pitch |
| Number of trays | : 15 equispaced , 666.67 cm diameter |
Feed distribution nozzle : |
|
| CO2 inlet | 265 holes of 8 mm diameter |
| NH3 inlet | 440 holes of 8 mm diameter |
| Operating/ Design temperature | 188/210oC |
| Operating/ Design pressure | 155/170 Kg/cm2 g |
| Design pressure | 170 Kg/cm2 g |
| Joint efficiency = j | 0.85 |
| Allowable stress = f | 22.5 Kg/cm2 g |
| Capacity | 2100 MTPD |
| Density of NH3/CO2 at 188oC | 881.5387/809.29 Kg/m3 |
Concentration Vs Rate of reaction data for carbon dioxide:
| Concentration CA, Kgmole/m3 |
18.39 |
16.55 |
14.71 |
12.87 |
11.03 |
9.19 |
7.35 |
5.51 |
| Rate of reaction, -rA Kgmole/hr m3 |
27.05 |
21.92 |
17.31 |
13.25 |
9.74 |
6.76 |
4.33 |
2.43 |
Calculation:
τ/CAo = V/fAo = ΔXA/ -rA
From material balance :
fAo = 2278.645 Kg mole/hr
CAo = fAo /Vo
Vo = (inlet flow of CO2)/(Density of CO2) = 100260.42 / 809.29 = 123.886 m3/hr
CAo = 2278.645/123.886 = 18.39 Kg mole/m3
Plotting graph (1/-rA) Vs CA :
| Concentration CA Kgmole/m3 |
18.39 | 16.55 | 14.71 | 12.87 | 11.03 | 9.19 | 7.35 | 5.51 |
| Rate of reaction -rA Kgmole/hr m3 |
27.05 | 21.92 | 17.31 | 13.25 | 9.74 | 6.76 | 4.33 | 2.43 |
| 1/-rA | 0.037 | 0.046 | 0.058 | 0.075 | 0.102 | 0.148 | 0.231 | 0.411 |
From graph :
Area = 137.8×2×0.02 = 5.512 hr
Area = τ = 5.512 hr
Now V = τ× fAo/CAo
V = (5.512×2278.65)/18.39 = 682.974 m3
Assuming height to be 18 meters
V = pi R2H
R2 = (683)/(π×10) = 12.075m2
R = 3.475 m
Diameter = 6.95 m
Types of Agitators
0
8:26 PM
Types of Agitators
 Propeller agitators
are commonly made of three bladed attached to the main shaft. They are
flexible in operations and mostly used in mechanical mixing of low to
medium viscosity fluids. These type of propellers are also called as
marine type propellers. The diameter of the propellers depends on the
rotational speed and diameter of the batch reactor or the agitator
vessel. Depending on the agitator vessel size and the fluid viscosity
the power consumption of the propeller agitator may exceed more than
50kW.
Propeller agitators
are commonly made of three bladed attached to the main shaft. They are
flexible in operations and mostly used in mechanical mixing of low to
medium viscosity fluids. These type of propellers are also called as
marine type propellers. The diameter of the propellers depends on the
rotational speed and diameter of the batch reactor or the agitator
vessel. Depending on the agitator vessel size and the fluid viscosity
the power consumption of the propeller agitator may exceed more than
50kW.

 Turbine impellers
operate at low speed and are much larger than propellers. Turbine has
an excellent feature in designing the flow patter where a change in
design can divert the flow pattern of fluid by radial flow or axial flow
in the reactor vessel. Based on the configuration of the impeller
blades these flow patterns can be achieved. Radial design make the fluid
to flow at high velocity in radial direction where as axial impellers
use pitched blades, make the fluid to flow parallel to shaft in downward
direction and then push the fluid towards the wall of the agitator
vessel. For gas dispersion operation radial turbine impeller is used and
axial turbine impeller is used for chemical reactions, suspension solid
and miscible liquid mixing.
Turbine impellers
operate at low speed and are much larger than propellers. Turbine has
an excellent feature in designing the flow patter where a change in
design can divert the flow pattern of fluid by radial flow or axial flow
in the reactor vessel. Based on the configuration of the impeller
blades these flow patterns can be achieved. Radial design make the fluid
to flow at high velocity in radial direction where as axial impellers
use pitched blades, make the fluid to flow parallel to shaft in downward
direction and then push the fluid towards the wall of the agitator
vessel. For gas dispersion operation radial turbine impeller is used and
axial turbine impeller is used for chemical reactions, suspension solid
and miscible liquid mixing. Types of agitators models, application and comparison:
| Agitator models | Application | Advantages | Disadvantages |
Paddle:
|
|
|
|
| Counter rotating paddles | Paste mixing |
|
|
| Tumbling | Blending | Paste and viscous material mixing | Not suitable for fluid solutions |
| Disk and cone | Polymers and dispersion preparation | Viscous solution mixing with 60 revolution per second | Paste mass cannot be handled |
| Free shaft suspension | Sugar processing | Suspension, Thickening operation | High power requirement |
| Impeller type | Emulsion preparations |
|
Not for viscous materials |
Turbine agitator
|
Liquid and gas reactions |
|
Only for less viscous liquid below 15 to 20 Ns/m2 |
Slotted rotary
|
Powders and cosmetics |
Unique particle size and homogeny product formation |
|
Screw
|
Food and snack processing | Homogenization of high viscous materials | Not suitable for miscibility operations |
Helical
|
Polymer and paints processing | Handles viscoelastic liquids that are more than 20 Ns/m2 | Less radial flow patterns |
| Gate | Blending operations |
|
Not for suitable for gas to liquid operations |
Anchor
|
Milk and fat processing | Efficient heat exchange between the reactor walls and reaction mass (fluids) |
|
| Propeller |
|
|
|
Industrial agitators
0
8:25 PM
Industrial agitators are machines used in industries that
process products in the chemical, food, pharmaceutical and cosmetic
industries, in a view of :
1.Anchor
2.Turbine
3.Propeller
4.Gas induction
Several different kind of industrial agitators exist:
These two phenomena provide energy consumption.
Turbines (flat blades or pitched blades) which inlet flow is axial and outlet flow is radial will provide shearing, turbulence and need approximately 20 time more energy than propellers, for the same diameter and same rotation speed.
If the operating conditions are under high pressure or high temperature, the agitator must be equipped with a sealing system to keep tightened the inside of the tank when the shaft is crossing it.
If the shaft is long (> 10m), it can be guided by a bearing located in the bottom of the tank (bottom bearing).
- mixing liquids together
- promote the reactions of chemical substances
- keeping homogeneous liquid bulk during storage
- increase heat transfer (heating or cooling)
Types
Mainly 4 types of Agitators are used in Pharmaceutical reactors, they are1.Anchor
2.Turbine
3.Propeller
4.Gas induction
Several different kind of industrial agitators exist:
- mechanical agitators (rotating)
- static agitators ( pipe fitted with baffles)
- Rotating tank agitators (like concrete mixer)
- Agitator Mixer Padole Type
- Agitators working with a pump blasting liquid
- Agitator turning thanks to gas
Principle of agitation
The agitation is achieved by movement of the heterogeneous mass(liquid-solid phase),to the impeller. This is due to mechanical agitators, to the rotation of an impeller. The bulk can be composed of different substances and the aim of the operation is to blend it or to improve the efficiency of a reaction by a better contact between reactive product. Or the bulk is already blended and the aim of agitation is to increase a heat transfer or to maintain particles in suspension to avoid any deposit.Data of an agitator
The agitation of liquid is made by one or several agitation impellers. Depending on its shape, the impeller can generate:- the moving of the liquid which is characterized by its velocity and direction.
- Turbulence which is an erratic variation in space and time of local fluid velocity.
- Shearing given by a velocity gradient between two filets of fluids.
These two phenomena provide energy consumption.
Impellers
Propellers (marine or hydrofoil) give an inlet and outlet which are on axial direction, preferably downward, they are characterized by a nice pumping flow, low energy consumption and low shear magnitude as well as low turbulence.Turbines (flat blades or pitched blades) which inlet flow is axial and outlet flow is radial will provide shearing, turbulence and need approximately 20 time more energy than propellers, for the same diameter and same rotation speed.
Mechanical features
An agitator is composed of a drive device ( motor, gear reducer, belts…), a guiding system of the shaft (lantern fitted with bearings), a shaft and impellers .If the operating conditions are under high pressure or high temperature, the agitator must be equipped with a sealing system to keep tightened the inside of the tank when the shaft is crossing it.
If the shaft is long (> 10m), it can be guided by a bearing located in the bottom of the tank (bottom bearing).
WHAT IS BOP
0
8:21 PM
This is an Overview about annular BOP (Hydrill).
Here, I am showing the description of hydrill and its parts by images.
this is the image that shows the internal building of hydrill,
and this is another model for annular
· Using a spherical rubber sealing element.
· Low one-piece height to save installation space.
· Large volume of spherical rubber sealing element, long service life.
· Wide sealing range, BOP can seal different sizes and shapes of pipe string, drill tools and wireline/cable not more than BOP bore size in diameter.
· Quick and reliable hydraulic drive.
· Low one-piece height to save installation space.
· Large volume of spherical rubber sealing element, long service life.
· Wide sealing range, BOP can seal different sizes and shapes of pipe string, drill tools and wireline/cable not more than BOP bore size in diameter.
· Quick and reliable hydraulic drive.
another type of BOP
Rotary BOP
The rotary blowout preventer (BOP) is used in balanced drilling, negative pressure (underbalance) drilling, counter circulation drilling and well drilling operation by using air, natural gas or foam as circulating medium as well as well servicing operation.
The self-sealing rubber sealing element can ensure that the rotary workover operation or the tripping in/out the pipe string operation with pressure borne is made under maximum kinetic pressure conditions.
Subscribe to:
Comments (Atom)











-Stack-2.jpg)


















